Sahar Forootan Sedigh, MS2 , Brian Gilmer, MD
Overview
Agili-C is a new, cutting-edge implant that consists of aragonite, a natural inorganic calcium carbonate mineral, that is used in cartilage and osteochondral defects1,2. It is a cell-free, (meaning there are no living cells in it) resorbable, (meaning it is absorbed by the body which it is implanted in) scaffold that is surgically implanted, causing the cartilage and bone cells in the region to affix themselves to the implant.
The hope is that this will allow for the growth and regeneration of the cartilage and underlying bone. During the surgical procedure, the surgeon cores out a cylinder of bone and cartilage which are damaged and places the Agili-C implant in its place. The implant is made of a material similar to coral that is found in the ocean, which is porous. The idea is that cells within the body will slowly migrate into and gradually replace the scaffold with fully new bone and an overlying cartilage layer of healthy tissue. Due to the implant being made of artificial material, there is theoretically less risk of patient rejection, (meaning the host body attacking the new implant). Agili-C was FDA approved in March 2022 and is one of the newest options for focal cartilage defects.
 Figure 1. Agili-C implant with micro-drilled channels on top.
Figure 1. Agili-C implant with micro-drilled channels on top.Image retrieved from: www.cartiheal.com/publications/
Indications
Articulate cartilage is smooth, white connective tissue that covers the ends of bones, acting as a shock absorber, and load distributor that allows for easy, painless movements. Normal cartilage is smoother than ice on ice.
Articular cartilage can be damaged due to injury/trauma. It does not have nerves, blood vessels, or lymph vessels that support its repair, therefore it’s very difficult for the cartilage to heal. This is the root of why cartilage repair and restoration is so difficult.
Once the damage occurs to the articulate cartilage, it becomes unsmooth causing the joints to rub against one another, triggering pain. This may start out as a focal lesion, meaning damage just in one area. Over time, damage to these surfaces can lead to arthritis, which is the widespread damage and degradation of the entire knee joint due to loss of the articular cartilage.
Osteochondral lesions occur when there is damage or a tear to the cartilage and the underlying bone – osteo = bone, chondral= cartilage. Physical injury, sports, diseases, trauma, and wear and tear can all be causes of osteochondral lesions. Agili-C has been proposed to repair this damage by generating growth of both bone and cartilage at the site of the lesion.
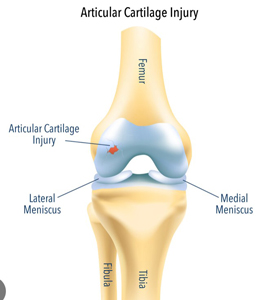 Figure 2. An injury to the surface of the articular cartilage is shown.
Figure 2. An injury to the surface of the articular cartilage is shown.Image retrieved from: www.vishalpai.com.au/articular-cartilage-injury
Symptoms
- Joint pain and swelling with activity
- Locking or clicking of the joint
- Dull ache of the joint
Implantation Procedure
Approach
Surgery begins by creating an incision at the joint to expose and mark the lesion. This approach is common to most types of cartilage repair and replacement technologies.
Sizing and Alignment
A perpendicular aligner is positioned in the center of the lesion and verified that it is perpendicular to the articulate surface. The implant should be surrounded by at least 3mm of bone. The implant comes in four sizes, 7.5mm, 10mm, 12.5mm, and 15mm, all having the same length of 10mm4.
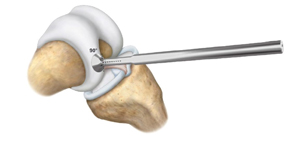 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
K-Wire
The Agili-C disposable mini-kit has a K-wire mounted a motorized drill. This wire goes through the perpendicular aligner and drilling into the lesion will occurs until the indicator line on the K-wire reaches the proximal end of the perpendicular aligner. The drill and the perpendicular aligner are removed with the K-wire still in place. This is the most important part of the surgery as it will be the foundation for the implantation site.
 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Drill Sleeve for the implantation site
A drill sleeve is placed over the K-wire with a drill bit attached to a motorized drill that will have a hole allowing it to slide over the K-wire. This step is creating the implantation site for the scaffold to be placed. It is what determines the size and depth of the implant.
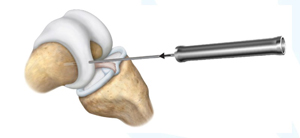 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
There will be a stop at the end of the drill bit once the correct depth has been reached. At this point, only healthy cartilage and bone will remain as the lesion will have been removed.
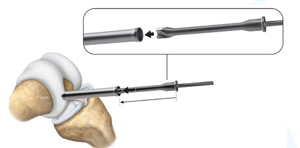 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Reamer and Shaper
A reamer is then placed over the K-wire that is manually rotated until the indicator line is leveled with the articular surface. Once the reamer is removed, the surgeon rinses the hole with saline to wash out any debris. The K-wire remains in place.
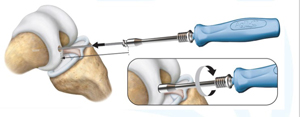 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
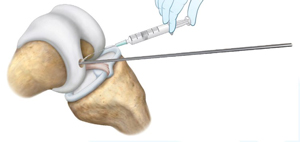 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
A shaper, which will shape the implantation site, is placed over the K-wire. It is manually rotated until the indicator line is leveled with the articular surface. The shaper rotates smoothly in the implantation site once it is fully sized. The shaper and K-wire are then removed.
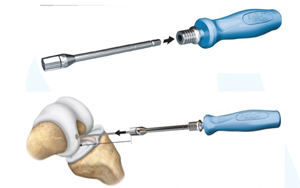 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Trimming the peripheral cartilage
A tool called the cartilage cutter will trim the peripheral cartilage to ensure smooth edges for implant insertion.
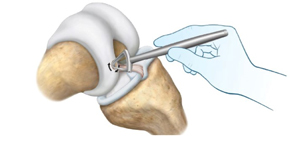 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Insertion of the Agili-C Implant
Gloves are changed prior to insertion. This is a good policy in general and is routine between different parts of an operation where different tissues or instruments are handled. The surgeon places the Agili-C implant into the implantation site. The surgeon pushed the implant in so that it is flush with the articular cartilage. It is important to verify that there is no entrapment of soft tissue and no cartilage invagination.
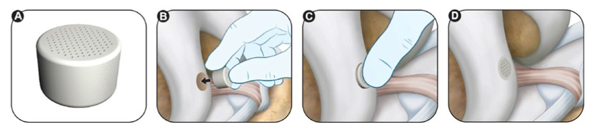 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Finally, a tamper is used to position the implant 2mm below the surface of the articular cartilage.
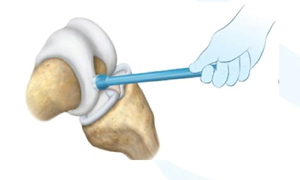 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
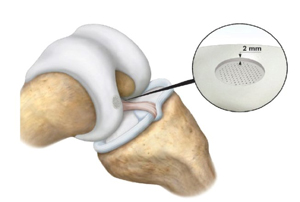 Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Image retrieved from: www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Risks the Agili-C implant
According to the manufacturer adverse effects include but are not limited to transient pain at the implant site, fever, bone marrow edema, reactive arthritis, aseptic arthritis, bone cyst, bone fracture bone deformity, ligament laxity, damage to meniscus, superficial or deep infections, deep vein thrombosis, and wound complications. These are the risks of most types of cartilage restoration procedures and fortunately are rare.
With Agili-C in particular there may be an allergic response in those that are allergic to materials containing calcium, calcium-carbonate, or coral. Additionally, Agili-C has not been studied in those that are younger than 22 so the outcome is unknown for younger population. It has not been tested in pregnant individuals, those that breastfeed, those that are receiving chemotherapy, have been diagnosed with osteoporosis, who are morbidly obese (BMI >35), and those that are immunocompromised to name a few.
From a study conducted on patients 2 years post-surgery, 7.2% (N=167) of the treatments failed. 4.8% of patients had their device removed. 0% had conversion to total knee replacement, which could be due to the short time frame post-surgery. This is in line with currently accepted and more common techniques for cartilage restoration.
Conclusion
Agili-C is a scaffold made of aragonite (similar to coral) that is used in cartilage and osteochondral defects for above the knee-join surface implants. It was approved by the FDA in March 2022. The implant has been used in hospitals in the United States, Belgium, Italy, Israel, Hungary, Poland, Romania, and Serbia. It is a new, promising procedure as it promotes the regeneration and growth of cartilage and bone. The implant will be dissolved by the body over time and the hope is that in its place will be healthy cartilage and bone.
Therefore, this implant may beneficial for those that have injured their articulate cartilage and are experiencing symptoms such as joint pain and swelling with activity, locking of the joint, and a dull ache of the joint. Agili-C implant can treat these concerns and allow for a healthy path towards the activities that you enjoyed doing prior to your injury.
Dr. Gilmer’s take-
Agili-C is brand new (2022), and I have not clinically adopted this technology into practice. I am waiting on further clinical data among other things. I have been following the clinical data and research with interest. In general, the hunt for restorative cartilage procedures continues. When talking about this goal, surgeons and researchers often refer to the regenerative triad: cells, scaffold, and signaling molecules. Right now, we can gather stem or progenitor cells, but without the other components, these stem cell treatments have not been reliable enough on their own. Agili-C works on the scaffold side, but there is a great deal we do not know about the migration of the host cells (the patient’s own cells), or the complex signaling cascade that orchestrates getting the right cells to the right place. To date, the biggest gap in our knowledge is within the signaling cascade that directs humans response to injuries. Stay tuned. We have a lot to learn on this topic.
It’s also important to note that Agili-C is for focal defects. The crucial difference between focal defects and arthritis is that these exist on a spectrum but all forms of cartilage injury seem to trigger an inflammatory cascade that is detrimental to the remaining cartilage. The gold standard treatment for arthritis remains osteotomy or some form of knee replacement, partial or total.
Brian Gilmer, MD – February, 2023
References
- Chubinskaya, S., Di Matteo, B., Lovato, L., Iacono, F., Robinson, D., & Kon, E. (2019). Agili-C implant promotes the regenerative capacity of articular cartilage defects in an ex vivo model.Knee Surgery, Sports Traumatology, Arthroscopy, 27(6), 1953-1964.
- Kon, E., Robinson, D., Verdonk, P., Drobnic, M., Patrascu, J. M., Dulic, O., ... & Filardo, G. (2016). A novel aragonite-based scaffold for osteochondral regeneration: early experience on human implants and technical developments. Injury, 47, S27-S32.
- Van Genechten, W., Vuylsteke, K., Struijk, C., Swinnen, L., & Verdonk, P. (2021). Joint surface lesions in the knee treated with an acellular aragonite-based scaffold: a 3-year follow-up case series.Cartilage, 13(1_suppl), 1217S-1227S.
- https://www.accessdata.fda.gov/cdrh_docs/pdf21/P210034C.pdf
Sahar Forootan Sedigh is a second-year medical student at the University of Nevada, Reno School of Medicine who conducted the background reading for this informational article.
* It should be noted that all information shared on this site is for informational purposes and seeks to curate other information available online and provide some context to how to interpret it. Any opinions expressed are intended as those of Dr. Gilmer based upon his clinical and research experience in the field of knee surgery. None of this constitutes medical advice and you should consult with a surgeon for more information when making medical decisions.








